Stephanie M. Lim1*,
Stephanie M. Lim1*,  Robert Wever2,
Robert Wever2,  Suzan D. Pas3,
Suzan D. Pas3,  Gygliola Bonofacio2,
Gygliola Bonofacio2,  Marion P. G. Koopmans3 and
Marion P. G. Koopmans3 and  Byron E. E. Martina1,3
Byron E. E. Martina1,3- 1Artemis One Health Research Foundation, Delft, Netherlands
- 2Medical Laboratory Services, Willemstad, Curaçao
- 3Department of Viroscience, WHO Collaborating Centre for Arboviruses and Hemorrhagic Fevers, Erasmus Medical Center, Rotterdam, Netherlands
Background: Zika virus (ZIKV) emerged in May 2015 in Brazil, from which it spread to many other countries in Latin America. Cases of ZIKV infection were eventually also reported in Curaçao (January 2016) and Bonaire (February 2016).
Methods: In the period of 16 December 2015 until 26 April 2017, serum, EDTA-plasma or urine samples were taken at Medical Laboratory Services (MLS) from patients on Curaçao and tested in qRT-PCR at the Erasmus Medical Centre (EMC) in the Netherlands. Between 17 October 2016 until 26 April 2017 all samples of suspected ZIKV-patients collected on Curaçao, as well as on Bonaire, were tested at MLS. Paired urine and/or serum samples from patients were analyzed for ZIKV shedding kinetics, and compared in terms of sensitivity for ZIKV RNA detection. Furthermore, the age and gender of patients were used to determine ZIKV incidence rates, and their geozone location to determine the spatial distribution of ZIKV cases.
Results: In total, 781 patients of 2820 tested individuals were found qRT-PCR-positive for ZIKV on Curaçao. The first two ZIKV cases were diagnosed in December 2015. A total of 112 patients of 382 individuals tested qRT-PCR-positive for ZIKV on Bonaire. For both islands, the peak number of absolute cases occurred in November 2016, with 247 qRT-PCR confirmed cases on Curaçao and 66 qRT-PCR-positive cases on Bonaire. Overall, a higher proportion of women than men was diagnosed with ZIKV on both islands, as well as mostly individuals in the age category of 25–54 years old. Furthermore, ZIKV cases were mostly clustered in the east of the island, in Willemstad.
Conclusions: ZIKV cases confirmed by qRT-PCR indicate that the virus was circulating on Curaçao between at least December 2015 and March 2017, and on Bonaire between at least October 2016 and February 2017, with peak cases occurring in November 2016. The lack of preparedness of Curaçao for the ZIKV outbreak was compensated by shipping all samples to the EMC for diagnostic testing; however, both islands will need to put the right infrastructure in place to enable a rapid response to an outbreak of any new emergent virus in the future.
Introduction
Zika virus (ZIKV) is an arbovirus that belongs to the Flaviviridae family, genus Flavivirus, and is transmitted through the bite of infected Aedes aegypti mosquitoes, via sexual contact (1–3), or from mother to fetus (4). ZIKV infection is often asymptomatic or otherwise presents with mild symptoms such as fever, macopapular rash, conjunctivitis, myalgia, and headache (5). In a small number of cases, ZIKV infection can result in serious complications such as Guillain-Barré syndrome (6–10), maculopathy (11–13), or microcephaly in newborns when the mother is infected with the virus during pregnancy (14–18).
Historically, since its discovery in Uganda in 1947, ZIKV was confined to Africa resulting only in sporadic cases of mild disease. In 2007, however, this pattern changed when the first major outbreak of ZIKV occurred in Yap (Federal States of Micronesia) where ~73% of the population was infected and symptomatic disease developed in ~18% of infected persons (19). Since then, ZIKV spread rapidly across the Pacific Ocean, causing outbreaks in French Polynesia (20), Cook Islands (20), Easter Island (21), New Caledonia (22), until eventually emerging in the Americas (23). Here it was first reported in Brazil in continental South America in May 2015, after which the virus spread to other Latin American countries, such as Colombia (October 2015), Surinam, El Salvador, Mexico, Guatemala, Paraguay, Venezuela (November 2015), Panama, Honduras, French Guiana, Martinique, Puerto Rico (December 2015), Maldives, Guyana, Ecuador, Barbados, Bolivia, Haiti, Saint Martin, Dominican Republic, Nicaragua, Jamaica, Curaçao, Costa Rica (January 2016), Bonaire and Aruba (February 2016) (24, 25). In Brazil alone, it has been estimated that between 440,000 and 1.3 million persons were infected with ZIKV in 2015 (26), and around 2366 cases of ZIKV-associated microcephaly/CNS malformations have been reported (as of February 2017, www.statista.com). Since then, the epidemic continued to spread, and the total number of infected persons and children with congenital ZIKV syndrome still remains to be determined.
Curaçao, a nation of almost 150,000 inhabitants, is known for its circulation of A. aegypti transmitted viruses, such as dengue virus (DENV) and chikungunya virus (CHIKV). DENV has been endemic on Curaçao for decades and outbreaks of the virus occur here every few years. The most recent outbreak of dengue occurred in 2014, where Curaçao health authorities had reported 194 suspected and 20 confirmed cases of dengue at the end of August (27). In June of the same year, the first case of CHIKV was also reported, which was the start of a major outbreak on the island that lasted until February 2015. By the end of November 2014, 1,838 suspected and 835 confirmed cases of CHIKV had been reported (28). Dengue is also endemic on Bonaire, a nation with almost 19,000 inhabitants, but not many reports are available.
Due to the high degree of serological cross-reactivity between flaviviruses, confirmation of infection poses a challenge. As IgM is thought to be more specific than IgG, detection of IgM against ZIKV by ELISA represents a possibility; however, cross-reactivity of DENV and ZIKV IgM has been demonstrated (29). This means that confirmative neutralization assays would still be required. As a result, confirmation of flavivirus infections is mostly based on detection of viral RNA in serum by using quantitative real-time PCR (qRT-PCR). However, for several arboviruses such as DENV or ZIKV, the level of viremia present in the blood during the symptomatic phase is often very low, which makes detection problematic. The use of urine as an alternative matrix for detecting ZIKV RNA was investigated by several laboratories and was found to be a good alternative to serum, EDTA-plasma and saliva, due to the higher levels of RNA found, and the longer period of time that urine was found positive after the onset of symptoms (>10–20 days) (30, 31). In contrast, another study demonstrated detection of ZIKV in whole blood for a longer period of time compared to urine and serum (32). Based on these observations, official World Health Organization (WHO) interim recommendations included using either whole blood, serum, or urine for nucleic acid testing (NAT), and serum for IgM detection (33). The routine confirmation of serological results by virus neutralization assays was not recommended as it was considered unfeasible.
To define the scope of the ZIKV outbreak on Curaçao and Bonaire, we determined the number of confirmed ZIKV cases based on qRT-PCR diagnostics, the incidence rates of infection in patients in terms of age and gender, as well as the geospatial distribution of ZIKV cases on Curaçao. In addition, paired urine samples from Curaçao were assessed for ZIKV shedding kinetics, while paired urine and serum samples from Bonaire were compared for sensitivity of ZIKV RNA detection.
Methods
In the period of 16 December 2015 until 26 April 2017, serum, EDTA-plasma or urine samples were taken at Medical Laboratory Services (MLS) from patients on Curaçao presenting with symptoms resembling ZIKV infection, such as fever, rash, headache or conjunctivitis. Between 16 December 2015 and 15 October 2016, the collected samples were inactivated and stabilized in MagnaPure lysis buffer (Roche Diagnostics, Almere, the Netherlands) and shipped to the diagnostic laboratory of the Erasmus Medical Centre (EMC) in Rotterdam, the Netherlands, where ZIKV RNA was tested by an ISO15189:2012 validated, internally controlled lab-developed real-time semi-quantitative qRT-PCR. In short, total nucleic acids were isolated using an external lysis protocol on the MagNA Pure LC robotics system (Roche Diagnostics) and subsequently tested in two independent qRT-PCRs using TaqMan® 1-Step Fast-Virus Master Mix (Thermo Fisher Scientific, Bleiswijk, the Netherlands) and primers targeting the envelope and the NS2A, in multiplex with an internal control (PDV), in a LC480-II cycler (Roche Life Science) (Table 1). The cut-off was set at <45 CT values. Starting from 6 July 2016, only the primer pair targeting the envelope was used in the qRT-PCR for confirmation of ZIKV infection.
As the etiology of the clinical manifestations of patients was still uncertain during the first 2 months of the outbreak (December 2015 and January 2016), serum samples collected from patients were also tested for DENV and CHIKV RNA using FTD Dengue/Chik multiplex (Fast Track Diagnostics, Esch-sur-Alzette, Luxembourg). Between December 2015 and October 2016 paired urine samples (plasma if urine was not available) with a target interval of ~2 weeks were submitted for testing. Starting from February 2016, either urine (matrix of choice) or EDTA-plasma samples (if urine was not available) were collected from patients.
In the period of 17 October 2016 until 26 April 2017, after the implementation of commercial ZIKV diagnostic assays at MLS, all samples of suspected ZIKV patients on Curaçao were collected and tested at MLS. In this period, samples were also collected from ZIKV-suspected patients on Bonaire by Fundashon Mariadal and sent to MLS for testing. In contrast to Curaçao, here it was chosen to collect paired serum and urine samples on the same day, from a large number of patients. The ZIKV diagnostic tests consisted of qRT-PCR and/or IgM/IgG ELISA (Euroimmun, Lübeck, Germany). For qRT-PCR, total nucleic acids were isolated using the MagNA Pure robotics system (Roche Diagnostis) and tested in a qRT-PCR using FTD Zika virus multiplex (Fast Track Diagnostics). Depending on the number of days after the onset of symptoms at which the patient was submitted for testing, the choice was made for either qRT-PCR alone (0–7 days), qRT-PCR and serology (7–14 days), or serology alone (≥14 days). However, as neither an ELISA-positive IgM or IgG result for ZIKV in a DENV-endemic area can be considered reliable due to the cross-reactivity known to exist between DENV and ZIKV antibodies (36, 37), we only considered positive results obtained in the qRT-PCR for the analyses.
In the period of 17 October 2016 up to 8 November 2016, plasma samples were tested, but from 9 November 2016 onwards, serum was chosen over EDTA-plasma due to its superior practicality and durability in the lab, and recommendations made by the World Health Organization (33). Urine was no longer the matrix of choice as serum could be used in both qRT-PCR and ELISA.
Curaçao can be divided into 65 geozones, which consist of one or more neighborhoods. The patients’ geozone of residence was used as a proxy for location and plotted on a map of Curaçao using www.mapcustomizer.com. The ZIKV incidence rates were determined for different age categories and the gender of patients.
Information such as presenting symptoms, day of onset, and pregnancy was not properly documented by the general practitioners on either Curaçao or Bonaire, and as a result, this data could not be included in the analyses. Written consent was provided by each individual submitting a urine, serum or plasma sample for testing, and written consent for children under 16 years of age was provided by their parent or guardian. As samples of patients were only collected for diagnostic purposes, no additional ethical clearance was required for this study.
Statistical Analyses
Paired samples were analyzed using a two-tailed paired t-test and P-values equal to or less than 0.5 were considered to be statistically significant.
Results
Between 16 December 2015 and 26 April 2017, 3,833 samples of 2,820 individuals were collected by MLS on Curaçao. Of these, 2,044 samples belonging to 1,685 patients were tested in qRT-PCR, resulting in 815 qRT-PCR positive samples, consisting of 781 positive ZIKV patients (Table 2). Testing of serum samples of ZIKV-suspected patients on Curaçao was first initiated in December 2015, during which two patients tested positive for ZIKV using qRT-PCR. During the first 2 months of the outbreak (December 2015 and January 2016), when serum samples were also tested in DENV and CHIKV qRT-PCRs, four out of 87 ZIKV-suspected disease cases were confirmed as positive for DENV instead (CT 26.6, 14.5, 29.5, and 34.4).
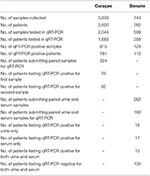 Table 2. Number of samples collected and tested, and the number of patients tested in qRT-PCR during the ZIKV outbreak on Curaçao and Bonaire.
Table 2. Number of samples collected and tested, and the number of patients tested in qRT-PCR during the ZIKV outbreak on Curaçao and Bonaire.
Of the 324 patients that submitted paired urine samples between December 2015 and October 2016, 70 patients tested positive for their first sample, while only 32 people still tested qRT-PCR-positive for their second sample (Table 2), taken between 11 and 17 days after the initial sample. This indicates that for some patients in this cohort, ZIKV RNA was still detectable in urine for up to 17 days. Furthermore, there was a significant trend in the decrease in the amount of virus shed in the urine over this time period (P < 0.0001, paired t-test) (Figure 1). No significant differences were found in the amount of virus shed in the urine between men and women (data not shown).
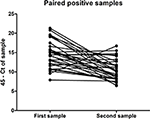 Figure 1. Amount of ZIKV RNA detected in the first and second ZIKV-positive urine samples of the paired samples submitted for testing by 32 individuals, expressed in terms of CT threshold 45 minus the CT determined for the sample.
Figure 1. Amount of ZIKV RNA detected in the first and second ZIKV-positive urine samples of the paired samples submitted for testing by 32 individuals, expressed in terms of CT threshold 45 minus the CT determined for the sample.
During the period of 17 October 2016 until 26 April 2017, a total of 744 samples were also collected from 382 individuals on Bonaire and tested at MLS Curaçao. Of these, 599 samples belonging to 358 patients were tested by qRT-PCR. A total of 129 samples consisting of 112 patients tested qRT-PCR positive for ZIKV. Of the 262 patients that had both a serum and urine sample taken on the same day, 183 had both samples concomitantly tested in qRT-PCR. Of these, 13 patients were positive for both serum and urine, while 17 patients tested positive for only serum, and 18 for only urine. One hundred 35 patients tested negative for both (Table 2).
For both islands, the peak number of absolute cases occurred in November 2016, with 247 qRT-PCR confirmed cases on Curaçao (Figure 2A) and 66 qRT-PCR-positive cases on Bonaire (Figure 2C; Table 3). In terms of prevalence, for Curaçao, the peak (79%) also occurred in November 2016 (Figure 2B), whereas for Bonaire the peak prevalence (50%) was in October 2016 (Figure 2D; Table 3). Overall, a higher proportion of women than men was diagnosed (~73%) on both Curaçao and Bonaire (Table 4), with incidence rates of 737 and 875 per 100,000, respectively. Furthermore, ZIKV was diagnosed mostly in individuals in the age category of 25–54 years old on both Curaçao (61%; incidence rate of 863 per 100,000) and Bonaire (67%; incidence rate of 815 per 100,000) (Table 4).
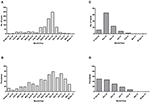 Figure 2. Absolute number of cases and prevalence of ZIKV-positive patients confirmed by qRT-PCR on Curaçao from mid-December 2015 to late April 2017 (A,B), and on Bonaire from mid-October 2016 to late April 2017 (C,D).
Figure 2. Absolute number of cases and prevalence of ZIKV-positive patients confirmed by qRT-PCR on Curaçao from mid-December 2015 to late April 2017 (A,B), and on Bonaire from mid-October 2016 to late April 2017 (C,D).
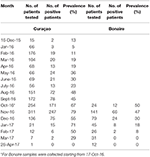 Table 3. Prevalence per month of qRT-PCR-confirmed ZIKV-positive patients on Curaçao and Bonaire during the outbreak.
Table 3. Prevalence per month of qRT-PCR-confirmed ZIKV-positive patients on Curaçao and Bonaire during the outbreak.
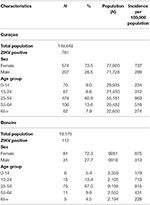 Table 4. Characteristics of the 781 patients confirmed by qRT-PCR for ZIKV infection on Curaçao between 16 December 2015 till 26 April 2017, and of the 112 patients confirmed on Bonaire between 17 October 2016 till 26 April 2017, according to sex and age [with use of population demographics data from July 2017 (www.indexmundi.com)].
Table 4. Characteristics of the 781 patients confirmed by qRT-PCR for ZIKV infection on Curaçao between 16 December 2015 till 26 April 2017, and of the 112 patients confirmed on Bonaire between 17 October 2016 till 26 April 2017, according to sex and age [with use of population demographics data from July 2017 (www.indexmundi.com)].
To determine the distribution of ZIKV infections on Curaçao, the locations of patients that tested positive for ZIKV by qRT-PCR were plotted on a map of Curaçao. Locations of 197 patients could not be pinpointed on the map. The map shows that the majority of the ZIKV cases were clustered in the eastern part of the island, particularly in Willemstad (Figure 3). Geozones with a notable number of infections included Santa Rosa, Spaanse Water, St. Michiel, Dominguito, Brievengat, Berg Altena, Tera Cora, Stenen Koraal, and Groot Piscadera.
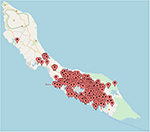 Figure 3. The locations of a selection of the patients on Curaçao that tested positive for ZIKV by qRT-PCR. The map was created by plotting the locations on www.mapcustomizer.com.
Figure 3. The locations of a selection of the patients on Curaçao that tested positive for ZIKV by qRT-PCR. The map was created by plotting the locations on www.mapcustomizer.com.
Discussion
Despite the documented emergence of ZIKV into the Americas in Brazil in May 2015, phylogenetic analyses estimate the introduction of the virus to be earlier, either between August 2013 and July 2014 (38) or between May and December 2013 (39). On Curaçao, according to our analyses, the first cases of ZIKV were diagnosed in December 2015, a month before the first notification to the WHO on 28 January 2016 (24), which indicates that the virus, most likely introduced by travelers, emerged earlier than officially reported. Given the rapid spread of the virus throughout the Americas after its emergence in Brazil, Curaçao, and Bonaire were not prepared for an outbreak of ZIKV, and diagnostic assays had therefore not yet been implemented and validated at MLS. This problem was circumvented by shipping patient samples to the diagnostic laboratory of the EMC in the Netherlands, a WHO Collaborating Centre for arboviruses. Starting from October 2016, MLS Curaçao had implemented the necessary commercial diagnostic qRT-PCR assay and ELISAs in order to continue the diagnosis of ZIKV-suspected patients on Curaçao and start with the diagnostics for Bonaire. Of note, this study was not designed prospectively but performed in reaction to a dynamic outbreak situation.
During this period, a switch was also made from urine to serum for samples collected on Curaçao. Even though a few studies have shown that urine was more sensitive for detection of ZIKV by qRT-PCR compared to serum (30, 31), the data from the paired serum and urine samples from Bonaire suggest that in this cohort, these two matrices were required concomitantly to increase the chance of ZIKV detection. As a result, it is possible that many ZIKV cases on both Curaçao and Bonaire were missed as here, paired urine and serum samples were not consistently collected and/or tested in qRT-PCR. Even though many PCR-negative samples from Curaçao and Bonaire had also been tested in IgM/IgG ELISA, the cross-reactivity known to occur between ZIKV and DENV antibodies makes diagnosis based on serology difficult (36, 37) and could easily lead to false positives. As a result, serology data of samples from patients collected 14 days after onset of symptoms were not included in our analyses, and our results are therefore very likely an underrepresentation of the number of ZIKV cases on both islands. Another factor that may have led to an underrepresentation of the total number of cases is the fact that not all individuals that experienced symptoms went to the general practitioner to get tested. Furthermore, on Curaçao, three laboratories were involved in the diagnostic testing of ZIKV patients, namely MLS, Analytisch Diagnostisch Centrum (ADC) and Laboratorio de Medicos (LabdeMed). If all the data were to be combined, the total number of ZIKV cases would likely be much larger than presented in this article.
The peak of the ZIKV outbreak on Curaçao appeared to occur in November 2016, both in terms of the absolute number of cases and prevalence. For Bonaire, the peak in the absolute number of cases seemed to occur in November 2016 as well, while in terms of prevalence it appeared to occur in October 2016. However, as no ZIKV diagnostics was carried out for Bonaire between mid-December 2015 and mid-October 2016, the data from October is not reliable for comparison with the other months, and it can also not be excluded that a larger number of people on Bonaire may have been infected in one of the months preceding November.
Interestingly, during the reported outbreak of ZIKV on Curaçao and Bonaire, no cases of microcephaly or fatalities due to ZIKV were reported. However, assuming a similar microcephaly risk of 0.02% for pregnant women as calculated for Brazil (40), and a fertility rate of ~2.1 for Curaçao [based on data from 2011 (41)], which is equivalent to ~2,100 live births per year, this would have given 0.42 cases of microcephaly during the outbreak on Curaçao (which lasted approximately a year). It is therefore not surprising that no cases of ZIKV-related microcephaly were observed in a population of only 150,000 and 19,000 people.
During the outbreak of ZIKV on both Curaçao and Bonaire, almost three times more women than men were infected with the virus. Infections occurred mostly in the age category of 25–54 years old for both men and women. This higher proportion of female infections during a ZIKV outbreak was also reported in Surinam (42) and Rio de Janeiro in Brazil (43). This disproportionate infection rate may be explained by the increased testing of pregnant women due to the concerns about microcephaly and other risks for the unborn babies. However, such a trend was also demonstrated in Rio de Janeiro during a DENV outbreak (43), where women were 30% more likely to be diagnosed with DENV than men. One explanation suggested by this study was that women are more conscientious about their health and therefore more likely to visit a general practitioner. Nonetheless, another possibility, as also speculated upon in the Coelho study (43), is that for ZIKV, a higher amount of male-to-female sexual transmissions occur in comparison to female-to-male transmissions. Infection of females by ZIKV via semen has already been demonstrated (1–3), and even though ZIKV has also been detected in the female genital tract and vaginal secretions (44–47), the ability of the virus to productively infect males via vaginal secretions during sexual intercourse has not yet been demonstrated. Furthermore, the influence of female reproductive hormones on ZIKV replication and transmission should also be investigated, as progestins have recently been shown to promote infection of HIV within the female reproductive tract of non-human primates (48).
In order to obtain an impression of the distribution of the number of ZIKV cases on Curaçao, the locations of the patients were plotted on a map. The majority of the cases were located in the east of the island, which may be the result of a reporting bias caused by a higher population density in the east (Willemstad) (41). Nonetheless, for geozones that contained the largest amount of ZIKV cases, no particular trend in terms of population density or average gross monthly income per household was identified (data not shown). It is possible that the geospatial distribution of ZIKV cases is a reflection of the presence of ZIKV-infected mosquitoes; however, as many inhabitants of Curaçao travel to different parts of the island on a daily basis, it is not possible to determine with certainty the location of transmission. Besides mosquito transmission, sexual transmission of ZIKV may also have influenced the geospatial distribution of cases on the island.
Conclusions
As Curaçao and Bonaire are (potential) hot-spots for emerging and re-emerging arbovirus infections, it is important that the islands are prepared for future outbreaks by implementing the appropriate diagnostic tools in advance. However, in addition to effective diagnostics, it is imperative that the right infrastructure is also put in place to allow communication during an outbreak setting and to facilitate the implementation of risk-reduction activities in order to deal with any infectious disease that may emerge in the future.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to patient privacy rights but a selection of datasets are available from the corresponding author on reasonable request.
Ethics Statement
Written consent was obtained from each individual that provided urine, serum or plasma samples. Consent for children under 16 years of age was provided by their parent or guardian. As MLS and the department of Viroscience are mandated to provide laboratory support for outbreak investigations, no additional ethical clearance was sought out.
Author Contributions
RW, SP, and GB coordinated and supervised the laboratory diagnostics and logistics. SL, SP, and GB were involved with the analyses. SL, SP, MK, and BM wrote the manuscript.
Funding
The research leading to these results has received funding from COMPARE (European Union’s Horizon 2020, Grant Agreement No. 643476), ZikaRisk (NWO ZonMW Project No. 522003001), and ZIKAlliance (European Union’s Horizon 2020, Grant Agreement No. 734548). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank all the laboratory personnel and staff members of MLS and the department of Viroscience for their technical and organizational contributions.
Abbreviations
ZIKV, Zika virus; DENV, dengue virus; CHIKV, chikungunya virus; IgM, immunoglobulin M; IgG, immunoglobulin G; ELISA, enzyme-linked immunosorbent assay; qRT-PCR, quantitative real-time polymerase chain reaction; RNA, ribonucleic acid; EDTA, ethylenediaminetetraacetic acid; WHO, World Health Organization; NAT, nucleic acid testing; MLS, Medical Laboratory Services; EMC, Erasmus Medical Center; NS2A, non-structural protein 2A; PDV, phocine distemper virus; CT, cycle threshold; FTD, Fast Track Diagnostics; ADC, Analytisch Diagnostisch Centrum; HIV, human immunodeficiency virus.
References
1. Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. (2011) 17:880–2. doi: 10.3201/eid1705.101939
2. Hills SL, Russell K, Hennessey M, Williams C, Oster AM, Fischer M, et al. Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission – continental United States, 2016. MMWR Morb Mortal Wkly Rep. (2016) 65:215–6. doi: 10.15585/mmwr.mm6508e2
3. D’Ortenzio E, Matheron S, Yazdanpanah Y, de Lamballerie X, Hubert B, Piorkowski G, et al. Evidence of sexual transmission of Zika virus. N Engl J Med. (2016) 374:2195–8. doi: 10.1056/NEJMc1604449
4. Calvet G, Aguiar RS, Melo ASO, Sampaio SA, de Filippis I, Fabri A, et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis. (2016) 16:653–60. doi: 10.1016/S1473-3099(16)00095-5
5. Galán-Huerta KA, Rivas-Estillaa AM, Martinez-Landerosb EA, Arellanos-Sotoab D, Ramos-Jiménez J. The Zika virus disease: an overview. Med Univ. (2016) 18:115–24. doi: 10.1016/j.rmu.2016.05.003
6. Oehler E, Watrin L, Larre P, Leparc-Goffart I, Lastere S, Valour F, et al. Zika virus infection complicated by Guillain-Barre syndrome–case report, French Polynesia, December 2013. Euro Surveill. (2014) 19:20720. doi: 10.2807/1560-7917.ES2014.19.9.20720
7. Cao-Lormeau VM, Blake A, Mons S, Lastère S, Roche C, Vanhomwegen J, et al. Guillain-Barre syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. (2016) 387:1531–9. doi: 10.1016/S0140-6736(16)00562-6
8. Roze B, Najioullah F, Fergé JL, Dorléans F, Apetse K, Barnay JL, et al. Guillain-barre syndrome associated with Zika virus infection in Martinique in 2016: a prospective study. Clin Infect Dis. (2017) 65:1462–8. doi: 10.1093/cid/cix588
9. Nascimento OJM, da Silva IRF. Guillain-Barre syndrome and Zika virus outbreaks. Curr Opin Neurol. (2017) 30:500–7. doi: 10.1097/WCO.0000000000000471
10. Araujo LM, Ferreira ML, Nascimento OJ. Guillain-Barre syndrome associated with the Zika virus outbreak in Brazil. Arq Neuropsiquiatr. (2016) 74:253–5. doi: 10.1590/0004-282X20160035
11. Yepez JB, Murati FA, Pettito M, Peñaranda CF, de Yepez J, Maestre G, et al. Ophthalmic manifestations of congenital Zika syndrome in colombia and venezuela. JAMA Ophthalmol. (2017) 135:440–5. doi: 10.1001/jamaophthalmol.2017.0561
12. Kodati S, Palmore TN, Spellman FA, Cunningham D, Weistrop B, Sen HN. Bilateral posterior uveitis associated with Zika virus infection. Lancet. (2017) 389:125–6. doi: 10.1016/S0140-6736(16)32518-1
13. Wong CW, Ng SR, Cheung CMG, Wong TY, Mathur R. Zika-related maculopathy. Retin Cases Brief Rep. (2019) 13:171–3. doi: 10.1097/ICB.0000000000000552
14. Miranda-Filho DDB, Martelli CM, Ximenes RA, Araújo TV, Rocha MA, Ramos RC, et al. Initial description of the presumed congenital Zika syndrome. Am J Public Health. (2016) 106:598–600. doi: 10.2105/AJPH.2016.303115
15. de Araujo TVB, Rodrigues LC, de Alencar Ximenes RA, de Barros Miranda-Filho D, Montarroyos UR, de Melo APL, et al. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: preliminary report of a case-control study. Lancet Infect Dis. (2016) 16:1356–63. doi: 10.1016/S1473-3099(16)30318-8
16. Rubin EJ, Greene MF, Baden LR. Zika Virus and microcephaly. N Engl J Med. (2016) 374:984–5. doi: 10.1056/NEJMe1601862
17. Honein MA, Dawson AL, Petersen EE, Jones AM, Lee EH, Yazdy MM, et al. Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA. (2017) 317:59–68. doi: 10.1001/jama.2016.19006
18. Brasil P, Pereira JP Jr, Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med. (2016) 375:2321–34. doi: 10.1056/NEJMoa1602412
19. Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. (2009) 360:2536–43. doi: 10.1056/NEJMoa0805715
20. Roth A, Mercier A, Lepers C, Hoy D, Duituturaga S, Benyon E, et al. Concurrent outbreaks of dengue, chikungunya and Zika virus infections – an unprecedented epidemic wave of mosquito-borne viruses in the Pacific 2012-2014. Euro Surveill. (2014) 19:20929. doi: 10.2807/1560-7917.ES2014.19.41.20929
21. Tognarelli J, Ulloa S, Villagra E, Lagos J, Aguayo C, Fasce R, et al. A report on the outbreak of Zika virus on Easter Island, South Pacific, 2014. Arch Virol. (2016) 161:665–8. doi: 10.1007/s00705-015-2695-5
22. Dupont-Rouzeyrol M, O’Connor O, Calvez E, Daurès M, John M, Grangeon JP, et al. Co-infection with Zika and dengue viruses in 2 patients, New Caledonia, 2014. Emerg Infect Dis. (2015) 21:381–2. doi: 10.3201/eid2102.141553
23. Fauci AS, Morens DM. Zika virus in the Americas–yet another arbovirus threat. N Engl J Med. (2016) 374:601–4. doi: 10.1056/NEJMp1600297
24. Kindhauser MK, Allen T, Frank V, Santhana RS, Dye C. Zika: the origin and spread of a mosquito-borne virus. Bull World Health Organ. (2016) 94:675–86C. doi: 10.2471/BLT.16.171082
25. Pan American Health Organization/World Health Organization. Timeline of the emergence of Zika virus in the Americas. Washington, DC: Pan American Health Organization/World Health Organization (2016).
26. Ministério da Saúde. Protocolo de vigilância e resposta à ocorrência de microcefalia relacionada à infecção pelo vírus Zika. Brasília: Departamento de Vigilância das Doenças Transmissíveis, Núcleo de Comunicação/SVS (2015).
27. Pan American Health Organization/World Health Organization. PLISA: Health Information Platform for the Americas – Reported Cases of Dengue Fever in the Americas. Pan American Health Organization/World Health Organization. Available at: http://www.paho.org/data/index.php/en/mnu-topics/indicadores-dengue-en/dengue-nacional-en/252-dengue-pais-ano-en.html (accessed July 19, 2018).
28. Pan American Health Organization/World Health Organization. Number of Reported Cases of Chikungunya Fever in the Americas, by Country or Territory 2013-2014, Cumulative Cases, EW 49. (2014). Available at: https://www.paho.org/hq/dmdocuments/2014/2014-dec-05-cha-chikungunya-cases-ew-49.pdf (accessed November 14, 2018).
29. Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. (2008) 14:1232–9. doi: 10.3201/eid1408.080287
30. Gourinat AC, O’Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M. Detection of Zika virus in urine. Emerg Infect Dis. (2015) 21:84–6. doi: 10.3201/eid2101.140894
31. Lamb LE, Bartolone SN, Kutluay SB, Robledo D, Porras A, Plata M, et al. Advantage of urine based molecular diagnosis of Zika virus. Int Urol Nephrol. (2016) 48:1961–6. doi: 10.1007/s11255-016-1406-9
32. Lustig Y, Mendelson E, Paran N, Melamed S, Schwartz E. Detection of Zika virus RNA in whole blood of imported Zika virus disease cases up to 2 months after symptom onset, Israel, December 2015 to April 2016. Euro Surveill. (2016) 21:30269. doi: 10.2807/1560-7917.ES.2016.21.26.30269
33. World Health Organization. Laboratory testing for Zika virus infection, WHO/ZIKV/LAB/16.1 (2016).
34. Ferreira-de-Brito A, Ribeiro IP, Miranda RM, Fernandes RS, Campos SS, Silva KA, et al. First detection of natural infection of Aedes aegypti with Zika virus in Brazil and throughout South America. Mem Inst Oswaldo Cruz. (2016) 111:655–8. doi: 10.1590/0074-02760160332
35. Hoek RA, Paats MS, Pas SD, Bakker M, Hoogsteden HC, Boucher CA, et al. Incidence of viral respiratory pathogens causing exacerbations in adult cystic fibrosis patients. Scand J Infect Dis. (2013) 45:65–9. doi: 10.3109/00365548.2012.708942
36. Priyamvada L, Quicke KM, Hudson WH, Onlamoon N, Sewatanon J, Edupuganti S, et al. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc Natl Acad Sci USA. (2016) 113:7852–7. doi: 10.1073/pnas.1607931113
37. Felix AC, Souza NCS, Figueiredo WM, Costa AA, Inenami M, da Silva RMG, et al. Cross reactivity of commercial anti-dengue immunoassays in patients with acute Zika virus infection. J Med Virol. (2017) 89:1477–9. doi: 10.1002/jmv.24789
38. Metsky HC, Matranga CB, Wohl S, Schaffner SF, Freije CA, Winnicki SM, et al. Zika virus evolution and spread in the Americas. Nature. (2017) 546:411–5. doi: 10.1038/nature22402
39. Faria NR, Azevedo RDSDS, Kraemer MUG, Souza R, Cunha MS, Hill SC, et al. Zika virus in the Americas: early epidemiological and genetic findings. Science. (2016) 352:345–9. doi: 10.1126/science.aaf5036
40. Jaenisch T, Rosenberger KD, Brito C, Brady O, Brasil P, Marques ETA. Risk of microcephaly after Zika virus infection in Brazil, 2015 to 2016. Bull World Health Organ. (2017) 95:191–8. doi: 10.2471/BLT.16.178608
41. Ter Bals M. Demography of Curaçao – Publication Series Census 2011. Willemstad: Central Bureau of Statistics (2014).
42. Codrington J, Roosblad J, Baidjoe A, Holband N, Adde A, Kazanji M, et al. Zika virus outbreak in Suriname, a report based on laboratory surveillance data. PLoS Curr. (2018) 10. doi: 10.1371/currents.outbreaks.ff0f6190d5431c2a2e824255eaeaf339
43. Coelho FC, Durovni B, Saraceni V, Lemos C, Codeco CT, Camargo S, et al. Higher incidence of Zika in adult women than adult men in Rio de Janeiro suggests a significant contribution of sexual transmission from men to women. Int J Infect Dis. (2016) 51:128–32. doi: 10.1016/j.ijid.2016.08.023
44. Penot P, Brichler S, Guilleminot J, Lascoux-Combe C, Taulera O, Gordien E, et al. Infectious Zika virus in vaginal secretions from an HIV-infected woman, France, August 2016. Euro Surveill. (2017) 22:30444. doi: 10.2807/1560-7917.ES.2017.22.3.30444
45. Murray KO, Gorchakov R, Carlson AR, Berry R, Lai L, Natrajan M, et al. Prolonged detection of Zika virus in vaginal secretions and whole blood. Emerg Infect Dis. (2017) 23:99–101. doi: 10.3201/eid2301.161394
46. Tang WW, Young MP, Mamidi A, Regla-Nava JA, Kim K, Shresta S. A mouse model of Zika virus sexual transmission and vaginal viral replication. Cell Rep. (2016) 17:3091–8. doi: 10.1016/j.celrep.2016.11.070
47. Sanchez-Montalva A, Pou D, Sulleiro E, Salvador F, Bocanegra C, Treviño B, et al. Zika virus dynamics in body fluids and risk of sexual transmission in a non-endemic area. Trop Med Int Health. (2018) 23:92–100. doi: 10.1111/tmi.13019
Keywords: Zika virus, outbreak, laboratory, qRT-PCR, epidemiology, Curaçao, Bonaire
Citation: Lim SM, Wever R, Pas SD, Bonofacio G, Koopmans MPG and Martina BEE (2019) Zika Virus Outbreak on Curaçao and Bonaire, a Report Based on Laboratory Diagnostics Data. Front. Public Health 7:333. doi: 10.3389/fpubh.2019.00333
Received: 11 June 2019; Accepted: 25 October 2019;
Published: 12 November 2019.
Edited by:
Matthew H. Collins, Emory University, United States
Reviewed by:
Man-Qing Liu, Wuhan Centre for Disease Prevention and Control, China
José Eduardo Levi, University of São Paulo, Brazil
Copyright © 2019 Lim, Wever, Pas, Bonofacio, Koopmans and Martina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephanie M. Lim, [email protected]